R&D
Combination with High Productivity and Safety
- Disadvantages : Reduced Bone Regeneration Capacity and Osteoconductivity (About 40% Compared to Autologous Bone)
BMP Protein Extraction & Culture Technology
- Introduction of Production Technology for Bone Growth-Inducing Protein without Allogeneic Bone/Freeze-Dried Bone
Allogeneic Bone or Freeze-Dried Allogeneic Bone
- Advantages : BMP-Inducing Capacity, the Source for the Bone Regeneration Capacity of Allogeneic Bone
- Disadvantages : Limited Supply of Source Material (Cost Increase)
BMP Synthetic Bone
- Difficulties in Controlling BMP Protein Production
- Possible Adverse Effects Such as Bone Marrow Cancer due to Large Amount of Osteoblast Production
Strengthening of Stem Cell Self-Treatment
- No Adverse Side Effects of BMP Protein
- Possible Differentiation of Undifferentiated Stem Cells
- Possible Improvement for Regeneration Capacity of Tendon, Ligament, Blood Vessels, and Soft Tissues
- Technology Applicable to Other Areas
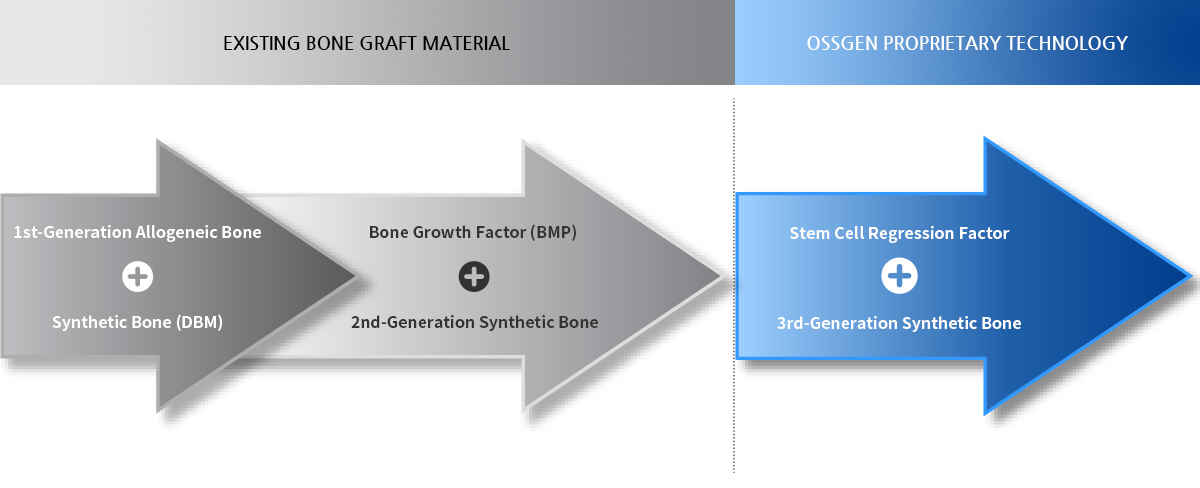
Bone Graft Material (Bone Grafting) Research Based on Allogeneic Bone and Synthetic Bone
-
Autologous Bone
- Limited Amount for Collecting Bones
- Increased Operation Time, Possible Complications
- Possible Defects in the Donor Bone
Xenogenic Bone
- Additional Processing is Needed After Collection
- Relatively Decreased Reconstruction Effect
- Possible Infection of Animal Diseases such as BSE (Mad Cow Disease)
-
-
Allogeneic Bone (Demineralized Bone Matrix, DBM)
- Contains Large Amounts of Growth Factors
- Excellent Biocompatibility
- Improved Approach of Reactive Cells and Improved Bone Regeneration-Inducing Capacity
Synthetic Bone
- Possible mass Production of Patient-Tailored Artificial Scaffolds
- Loading of Bioactive Materials / Control of Release Rate
- Fusion of Functional Cells
Development of Biocompatible Artificial Scaffolds through joint R&D and Securing of Core Technology with Excellent Promotion of Bone Formation
Bone Graft Material (Bone Grafting) Research Based on Allogeneic Bone and Synthetic Bone”
| Amnion : It is the Innermost thin Membrane of the Placenta at 0.03mm Thick, Containing Collagen Ⅲ, Ⅳ and Glycoproteins (Laminin, Fibronectin) that can Play the Role of Biological Scaffold. | Expected Synergistic Effects of Tissue Regeneration by Increasing Cell Adhesion using Collagen as Carrier. |
| No Harmful Effects to the Human body without Immune Responses | Containing Various Growth and Differentiation Factors |
| Anti-Inflammatory, Anti-Bacterial Effects |
To Aim for the Development of Diabetic Foot Ulcer Dressing Foam Preparation using the High Wound-Healing Capacity of Amnion and the Establishment of Amnion-Containing Functional Cosmetics Technology through Expanded Studies
Expansion of Possible Utilization of Collagen through the Reuse of Discarded Human Adipose
-
- The Reuse of Discarded Human Adipose, which has Been Classified as Medical waste and About 1,000 tons of which is Burned Annually, Becomes Possible within the Special Zone for Regulatory Freedom.
-
- Possible Production of Bio Materials Including Collagen, Extracellular Matrix (ECM), Adipose Stem Cells, and Hyaluronic Acid.
No Domestic Products Including Pharmaceuticals and Medical Devices Containing Human Collagen
Human Collagen is Excellent in Biocompatibility, Cell Proliferation, and Differentiation without Immune Rejection.
It has the Advantages of Convenient Allograft through the Elimination of Cellular and Immune Components.
R&D is in Progress to Aim for Original Technology for Medical Devices with Bio-Affinity Materials using Human Collagen.
R&D Pipelines for Product Enhancement and Diversification